
Validation N Decontamination
Customized Validation and Decontamination services as per your requirements
We deliver end-to-end validation and decontamination services for scientific and pharma equipment—covering risk assessment, protocols, execution, documentation, and audit support.
Request a Validation Plan





From single instruments to full facilities—protocols, execution, and documentation aligned with global regulations.

Customized Validation and Decontamination services as per your requirements

Validation of your lab equipments for optimizing their performance.

Regular validation not only helps maintain compliance but also reduces downtime, prevents data inaccuracies, and extends the lifespan of critical scientific instruments.
Pharma • Biotech • CRO • Food & Beverage • Diagnostics • Research Labs
Flow, Precision, Accuracy, Linearity, Carryover.

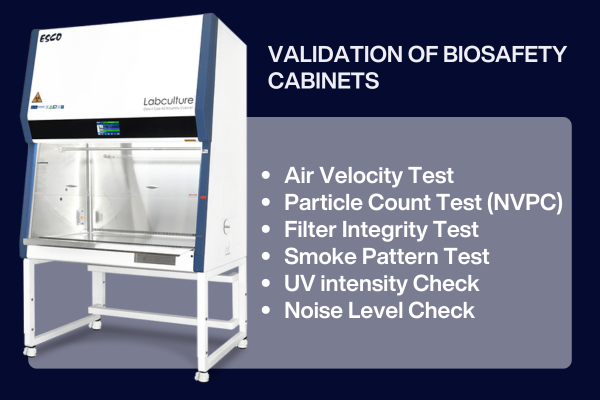
Clean, Unidirectional Airflow.

Airflow pattern, Duct integrity, and Containment.
Laboratory competence guidance for calibration & testing.
Validation planning consistent with Annex 15 & GAMP 5 principles.
Electronic records/signatures and data integrity controls.
Get clearance from the site to start validation process.
Clean the equipment thoroughly before start of validation.
Perform Validation as per Standard Operating Procedures (SOP).
Perform Decontamination for the equipment based on client's request..
Share your equipment list and we’ll build a validation plan.